JIANGSU TIANCHENG GROUP LIMITED
Yaoguan Town, Changzhou City, Jiangsu Province,China
+86-519-88387662
+86-519-85121683
steel_tube
steeltube.china
Tiancheng_Group
josen_dong
info@tiancheng.org
From all the metals humans ever used non of them created a revolution in technomogy at the scale Iron did. This metal became the reference element in metalurgically studies for other metals as well. If you understand Iron you actually understand not only metals but you understand the entire material science engineering. In this post I want to talk about it, for everyone who intend to start in materials science this is the 1st metal you will need to study and understand. It is exaclty how I also started to study materials. Iron was the first one for me too. Let’s see what’s its story.
The global economy has grown by leaps and bounds in the last couple of centuries and steel has had an indispensable role in this development. But what do we know of its origins?
To know steel, we must first understand iron (Fe)(from latin = ferrum), for these two metals are nearly one and the same. Steel contains 98-99% of iron concentration or more. The small remainder is carbon (C), which makes a major difference in the metal’s properties. Now let’s go back a little in time to know how it all started.
It is an odd fact that steel was not understood by science until the 20th century. Before that, for thousands of years, the making of steel was handed down through the generations as a craft. Even in the 19th century, when we had an impressive theoretical understanding of astronomy, physics and chemistry, the making of iron and steel on which our Industrial Revolution was based was achieved empirically – through intuitive guesswork, careful observation and a huge slice of luck. During the Stone Age, metal was extremely rare and highly prized, since the only sources of it on the planet were copper (Cu) and gold (Au), which occur naturally, if infrequently, in the Earth’s crust (unlike most metals, which have to be extracted from ores). Some iron (Fe) existed too, most of it having fallen from the sky in the form of meteorites.
In the absence of copper, gold and meteoric iron, our ancestors’ tools during the Stone Age were made of flint, wood and bone. Anyone who has ever tried to make anything with these kinds of tools knows how limiting they are: if you hit a piece of wood it either splinters, cracks or snaps. The same is true of rock or bone. Metals are fundamentally different from these other materials because they can be hammered into shape: they flow, they are malleable. Not only this, they get stronger when you hit them; you can harden a blade just by hammering it. And you can reverse the process simply by putting metal in a fire and heating it up, which will cause it to get softer. The first people to discover these properties 10,000 years ago had found a material that was almost as hard as a rock but behaved like a plastic and was almost infinitely reusable. In other words they had discovered the perfect material for tools, and in particular cutting tools like axes, chisels and razors. This ability of metals to transform from a soft to a hard material must have seemed like magic to our ancient ancestors.
In the late 18th century, during the Industrial Revolution in England, the invention of the steam engine by James Watt enabled the blasting of air into the blast furnace with a machine. This made the mass production of iron possible. Although iron was the main driver of this revolution, it was by no means a new material. It had been around for nearly 3000 years since the Iron Age. For many centuries, the British had converted their iron ores to iron & steel by heating the raw material with charcoal obtained from trees.
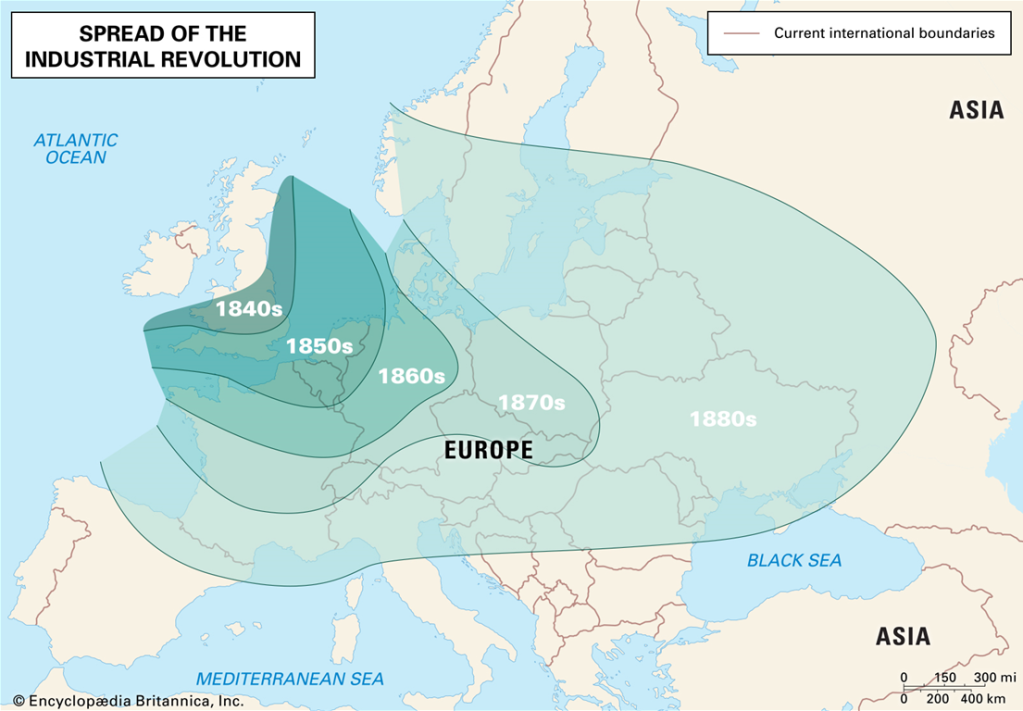
By the mid-18th century, however, the iron industry was in a downward trajectory. Iron workers required charcoal to smelt the iron ore, but charcoal was in short supply as the wood required to make charcoal was expensive. This posed an industrial problem. What was now required was a method by which iron could be smelted in high tonnage quantities. It needed a better heat source than charcoal, possibly a new fuel for treating iron ores.
The fuel they found was coal. In the course of a century, substantial change occurred as coal replaced charcoal as the fuel for smelting process. Coke proved to be a far superior material for converting iron ore to iron and then steel. It was obtained by heating coal in the absence of air. The materials were now cost efficient and sufficient in supply. The result of this change revolutionised the industry and the use of iron and steel.
The conversion of the iron and steel from charcoal to coke was accompanied, however, by several new technical problems, like the high concentration of sulphur (S) in coal, which along with other impurities makes iron brittle. Steel was a stronger and less brittle than iron, but it was more difficult to make. This encouraged the development of even more new inventions. Along the way, a key discovery was that the amount of carbon present in the iron controlled not only its melting point but also its properties. By controlling the additions of carbon (C) through the use of coke, a form of iron was made called steel which could be cast on an industrial scale.
At the beginning of 20th century steel was already notorious for the ability to keep its shape so it was started to be used for razor blades which were first introduced by the american inventor King Camp Gillette in 1901. In fact daily shaving was not a widespread practice in the 19th century so some people never shaved. The custom of shaving every day among American men is a 20th-century innovation which was started after World War I. During this time these razor blades indeed proved that they are very usefull and the next idea that came up was to build a kind of machine which could be used to sharpen these blades easier. But here things are not that simple as they seem. To build such a machine and to effectively measure the resulted sharpness we must have a real appreciaction about the phisics and chemistry taking place during the sharpening process.
The reason is that metals are made from crystals, the average razor blade contains billions of them, and in each of these crystals the atoms are arranged in a very particular way, a near-perfect three-dimensional pattern.
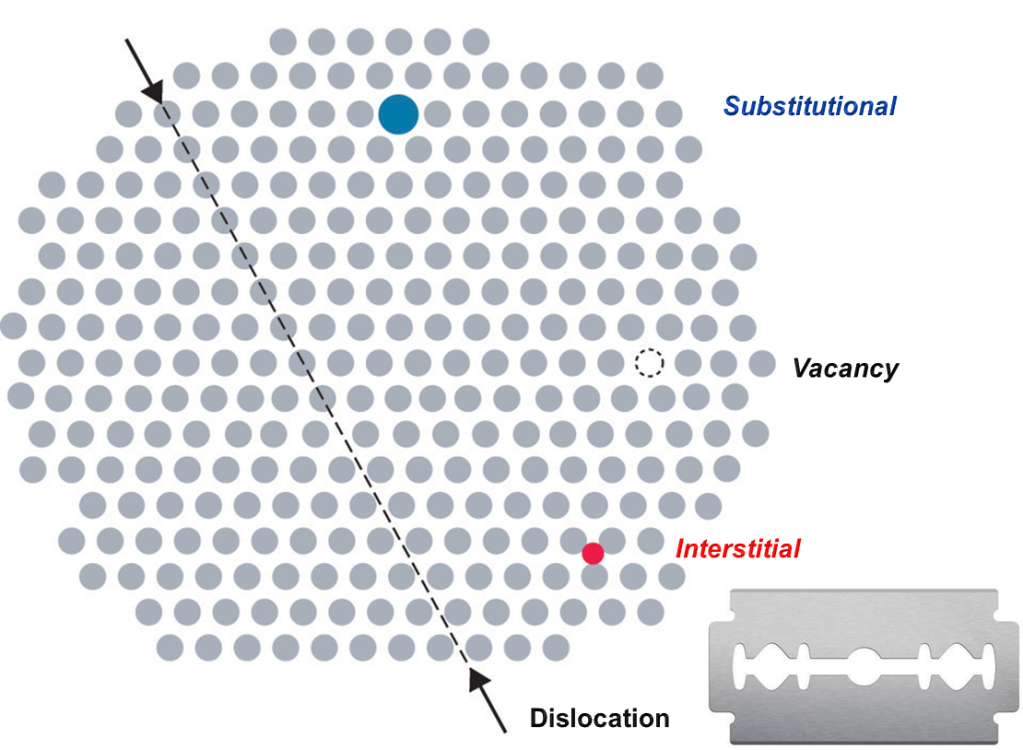
A metal crystal, such as exists inside a razor. The rows of dots represent atoms.
The bonds between the atoms hold them in place and also give the crystals their strength. A razor gets blunt because the many collisions with hair that it encounters force bits of these crystals to rearrange themselves into a different shape, making and breaking bonds and creating tiny dents in the smooth razor edge. Resharpening a razor through some electronic mechanism, would have to reverse this process. In other words, it would have to move atoms around to rebuild the structure that had been destroyed.
To sharpen a piece of something that means you must remove a layer of material form it and this action genereates heat. This heat, whether electrically produced or not, usually has a different effect on the material, it can change its atomic structure and it always softens metal crystals, therefore the sharpening process must avoid excessive heating.
It may be odd to think that metals are made of crystals because our typical image of a crystal is of a transparent and highly faceted gemstone such as a diamond or emerald. The crystalline nature of metals is hidden from us because metal crystals are opaque, and in most cases microscopically small. Viewed through an electron microscope, the crystals in a piece of metal look like crazy paving, and inside those crystals are squiggly lines – these are dislocations. They are defects in the metal crystals, and represent deviations in the otherwise perfect crystalline arrangement of the atoms – they are atomic disruptions that shouldn’t be there. They sound bad, but they turn out to be very useful. It is dislocations that make metals so special as materials for tools, cutting edges and ultimately the razor blade, because it is they that allow the metal crystals to change shape. You don’t’ need to use a hammer to experience the power of dislocations. When you bend a paper clip, it is in fact the metal crystals that are bending. If they didn’t bend, the paper clip would be brittle and snap like a stick. This plastic behavior is achieved by the dislocations moving within the crystal. As they move they transfer small bits of the material from one side of the crystal to the other. They do this at the speed of sound. As you bend a paper clip, you are causing approximately 100,000,000,000,000 dislocations to move at a speed of hundreds of meters per second. Although each one only moves a tiny piece of the crystal (one atomic plane in fact), there are enough of them to allow the crystals to behave like a super-strong plastic rather than a brittle rock.
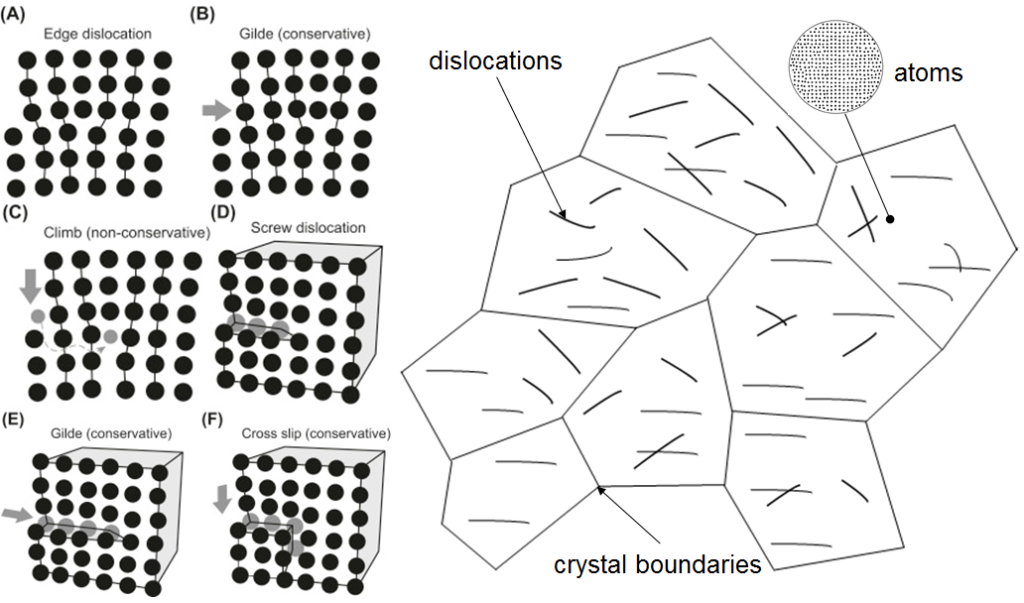
simplified crystalS REPRESENTATION with dislocation in their structure. Normal metals have enormous numbers of dislocations which overlap and intersect
The melting point of a metal is an indicator of how tightly the metal atoms are stuck together and so also affects how easily the dislocations move. Lead (Pb) has a low melting point and so dislocations move with consummate ease, making it a very soft metal. Copper (Cu) has a higher melting point and is stronger. Heating metals allows dislocations to move about and reorganize themselves, one of the outcomes of which is that it makes metals softer.
Discovering metals was an important moment in pre-history, but it didn’t solve the fundamental problem that there wasn’t very much metal around. One option, clearly, was to wait for some more to drop from the sky but this requires a huge amount of patience (a few kilograms fall on the surface of the Earth every year, but mostly into the oceans). But at some point someone made the discovery that would end the Stone Age and open the door to a seemingly unlimited supply of the stuff. They discovered that a certain greenish rock, when put into avery hot fire and surrounded by red-hot embers, turns into a shiny piece of metal. This greenish rock was malachite and the metal was, of course, copper (Cu). It must have been the most dazzling revelation. Suddenly they were surrounded not by dead inert rock but by mysterious stuff that had an inner life. They would have been capable of performing this transformation with only a few particular types of rock, such as malachite, because getting it to work reliably depends not just on identifying these rocks but also on carefully controlling the chemical conditions of the fire. But they must have suspected that those rocks that didn’t work, which remained obstinately rock-like however hot the fire became, had hidden secrets. They were right. It’s a process that works for many minerals, although it would be thousands of years before an understanding of the chemistry required (controlling the chemical reactions between the rock and the gases created in the fire) led to the next real breakthrough in smelting.
It the meantime, from around the year 5000 B.C., they used trial and error to hone the process of the production of copper. The making of copper tools initiated a spectacular growth in human technology, being instrumental in the birth of other technologies, cities and the first great civilizations. The pyramids of Egypt are an example of what became possible once there were plentiful copper tools. Each block of stone in each pyramid was extracted from amine and individually hand-carved using copper chisels. It is estimated that 10,000 tons of copper ore were mined throughout Ancient Egypt to create the 300,000 chisels needed. It was an enormous achievement, without which the pyramids could not have been built, however many slaves were used, since it is not practical to carve rock without metal tools. It is all the more impressive given that copper is not the ideal material for cutting rock since it is not very hard. Sculpting a piece of limestone with a copper chisel quickly blunts the chisel. It is estimated that the copper chisels would have needed to be sharpened every few hammer blows in order for them to be useful. Copper is not ideal for razor blades for the same reason.
Gold (Au) is another relatively soft metal, so much so that rings are very rarely made from pure gold metal because they quickly scratch. But if you alloy gold, by adding a few per cent of other metals, such as silver (Ag) or copper (Cu), you not only change the colour of the gold – silver making the gold whiter, and copper making the gold redder – you make the gold harder, much harder. This changing of the properties of metals by very small additions of other ingredients is what makes the study of metals so fascinating. In the case of gold alloys, you might wonder where the silver atoms go. The answer is that they sit inside the gold crystal structure, taking the place of a gold atom, and it is this atom substitution inside the crystal lattice of the gold that makes it stronger.
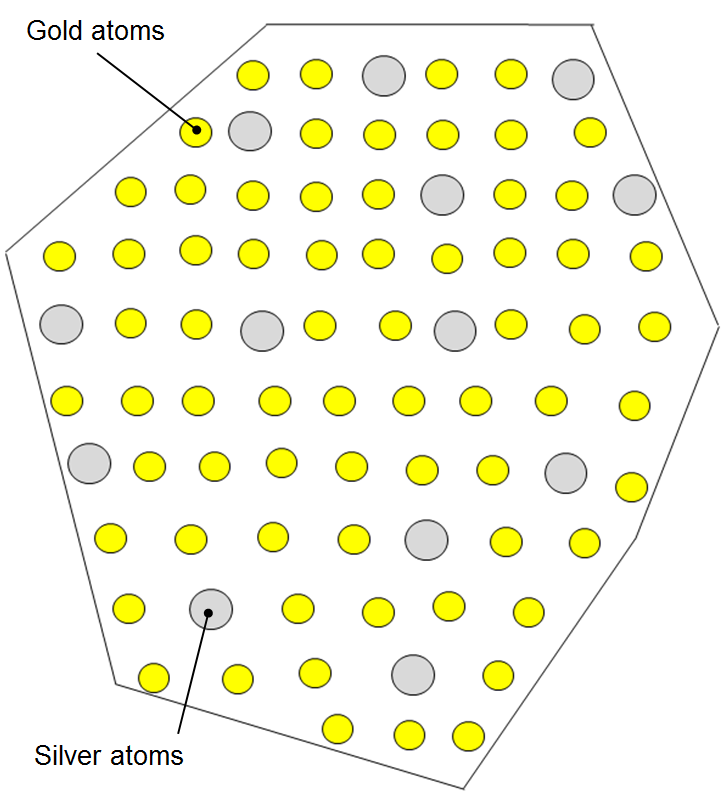
Gold alloyed with silver at the atomic scale, showing how the silver atoms replace some of the gold atoms in the crystal.
Alloys tend to be stronger than pure metals for one very simple reason: the alloy atoms have a different size and chemistry from the host metal’s atoms, so when they sit inside the host crystal they cause all sorts of mechanical and electrical disturbances that add up to one crucial thing: they make it more difficult for dislocations to move. And if dislocations find it difficult to move then the metal is stronger, since it’s harder for the metal crystals to change shape. Alloy design is thus the art of preventing the movement of dislocations.
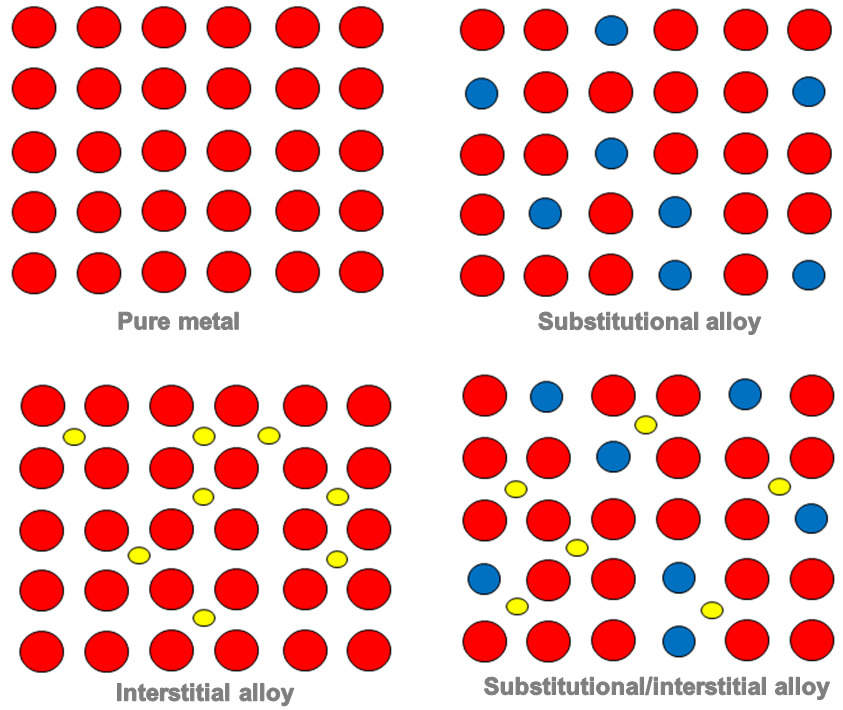
THE STRUCTURE OF a PURE METAL VERSUS ITS ALLOYS [yellow and blue are atoms of different metal(s) that the main one)]
These atom substitutions happen naturally inside other crystals too. A crystal of aluminium oxide is colourless if pure but becomes blue when it contains impurities of iron atoms: it is the gemstone called sapphire. Exactly the same aluminium oxide crystal containing impurities of chromium is the gem called ruby.
The ages of civilization, from the Copper Age to the Bronze Age to the Iron Age, represent a succession of stronger and stronger alloys. Copper is a weak metal, but naturally occurring and easy to smelt. Bronze is an alloy of copper, containing small amounts of tin (Sn) or sometimes arsenic (As), and is much stronger than copper. So, if you had copper and you knew what you were doing, for very little extra effort you could create weapons and razors 10 times stronger and harder than copper. The only problem is that tin and arsenic are extremely rare. Elaborate trade routes evolved in the Bronze Age to bring tin from places such as Cornwall and Afghanistan to the centres of civilization in the Middle East for precisely this reason.
Modern razors too are made from an alloy but, it is a very special sort of alloy, the existence of which puzzled our ancestors for thousands of years. Steel, the alloy of iron (Fe) and carbon (C), is even stronger than bronze, with ingredients that are much more plentiful: pretty much every bit of rock has some iron in it, and carbon is present in the fuel of any fire. Our ancestors didn’t realize that steel was an alloy – that carbon, in the form of charcoal, was not just a fuel to be used for heating and reshaping iron but could also get inside the iron crystals in the process. Carbon doesn’t do this to copper during smelting, nor to tin or bronze, but it does to iron. It must have been incredibly mysterious – and only now with a knowledge of quantum mechanics can we truly explain why it happens (the carbon in steel doesn’t take the place of an iron atom in the crystal, but is able to squeeze in between the iron atoms, creating a stretched crystal).
There is another problem, too. If iron becomes alloyed with too much carbon – if, for instance, it contains 4% carbon instead of 1% carbon – then it becomes extremely brittle and essentially useless for tools and weapons. This is a major obstacle because inside a fire there is rather a lot of carbon around. Leave the iron in too long, or allow it to become liquid in the fire, and a huge amount of carbon enters the metal crystals, making the alloy very brittle. Swords made from this high-carbon steel snap in battle.
Until the 20th century, when the alloying process was 1st fully explained, no one understood why some steel-making processes worked and others didn’t. They were established by trial and error, and those that were successful were handed down to the next generation and were often trade secrets. But even if they were stolen, they were so complicated that the chances of successfully reproducing someone else’s steel-making process were very low. Certain metallurgical traditions in certain cultures became known for making extremely high-quality steel-and such civilizations thrived.
In 1961 Professor Richmond from Oxford University discovered a pit that had been dug by the Romans in AD 89. It contained 763,840 small two-inch nails, 85,128 medium nails, 25,088 large nails, and 1,344 extra-large 16-inch nails. The hoard was of iron and steel and not gold, which most people would have found bitterly disappointing. But not Professor Richmond, Why, he asked himself, would a Roman legion bury 7 tonnes of iron and steel?
The Roman legion had been occupying the advanced head- quarters of Agricola in a place called Inchtuthil in Scotland. This was at the outer reaches of the Roman Empire and their mission was to protect its border from what they saw as the savage tribes who threatened it: the Celts. The legion of 5000 men occupied the region for 6 years before retreating and, in the process, abandoning their fort. They made great efforts to leave behind nothing that could help their enemies. They smashed all food and drink containers and burned the fort to the ground. But they weren’t satisfied with this. In the ashes were the steel nails that had held the fort together, and they were far too valuable to be left to the tribes that had driven them out. Iron and steel were the materials that enabled the Romans to build aqueducts, ships and swords; they allowed them to engineer an empire. Leaving the nails to their enemies would have been as useful as leaving a cache of weapons, so they buried them in a pit before marching south. As well as their weapons and armour, among the few, smaller steel objects that they probably did take with them were novacili, an object that epitomized their civilized approach to life: the Roman razor blade. These novacili, and the barbers who wielded them, allowed the Romans to retreat clean-shaven, groomed in order to distinguish themselves from the savage hordes that had driven them out.
The mystique that surrounded steel making engendered various myths, and the unification and restoration of order to Britain in the wake of the Roman retreat was symbolized by one of the most enduring of these: Excalibur, the legendary sword of King Arthur, sometimes attributed with magical powers and associated with the rightful sovereignty of Britain.

The Excalibur Sword_The Sword of King Arthur
At a time when swords regularly snapped in battle, leaving a knight defenseless, it is easy to see why a high-quality steel sword wielded by a strong warrior came to represent the rule of civilization over chaos. The fact that the process of making steel was, necessarily highly ritualized helps also to explain why this material came to be associated with magic.
This was nowhere more true than in Japan, where the forging of a Samurai blade took weeks and was part of a religious ceremony. The Ama-no-Murakumo-no- Tsurugi (‘Sword of the Gathering Clouds of Heaven’) is a legendary Japanese sword which allowed the great warrior Yamato Takeru to control the wind and defeat all his enemies. Despite the fantastic stories and rituals, the idea that some swords could be made 10 times stronger and sharper than other swords was not just a myth, but a reality. By the 15th century AD the sword steel made by the Samurai of Japan was the best the world had ever seen and remained pre-eminent for 500 years until the advent of metallurgy as a science in the twentieth century.

The Ama-no-Murakumo-no- Tsurugi –The Sword of the Gathering Clouds of Heaven
These Samurai swords were made from a special type of steel called tamahagane, which translates as ‘jewel steel’, made from the volcanic black sand of the Pacific (this consists mostly of an iron ore called magnetite, the original material for the needle of compasses). This steel is made in a huge clay vessel four feet tall, 4 feet wide and 12 feet long called a tatara. The vessel is ‘fired’ – hardened from moulded day into a ceramic – by lighting a fire inside it. Once fired, it is packed meticulously with layers of black sand and black charcoal, which are consumed in the ceramic furnace. The process takes about a week and requires constant attention from a team of 4 or 5 people, who make sure that the temperature of the fire is kept high enough by pumping air into the tatara using a manual bellows.
At the end the tatara is broken open and the tamahagane steel is dug out of the ash and remnants of sand and charcoal. These lumps of discolored steel are very unprepossessing but what makes them special is that they have a whole range of carbon content, some of it very low and some of it high. The Samurai innovation was to be able to distinguish high-carbon steel, which is hard but brittle, from low-carbon steel, which is tough but relatively soft. They did this purely by how it looked, how it felt in their hands and how it sounded when struck. By separating the different types of steel, they could make sure that the low-carbon steel was used to make the center of the sword. This gave the sword an enormous toughness, almost a chewiness, meaning that the blades were unlikely to snap in combat. On the edge of the blades they welded the high-carbon steel, which was brittle but extremely hard and could therefore be made very sharp. By using the sharp high-carbon steel as a wrapper on top of the tough low-carbon steel they achieved what many thought impossible: a sword that could survive impact with other swords and Armour while remaining sharp enough to slice a man’s head off. The best of both worlds.